文章来源:医脉通
2015年ASCO年会于5月29日—6月2日在美国芝加哥召开。5月30日下午消化系统(结直肠)肿瘤口头报告专场上,一项来自PETACC8和N0147试验3934例患者的汇总分析显示了,BRAF V600E与KRAS外显子2突变对经辅助FOLFOX+/-西妥昔单抗治疗,微卫星稳定(MMS),III期结肠癌患者的预测价值。医脉通整理如下:
既往发表的研究因混杂了II期与III期,微卫星不稳定性(MSI)和MSS,结肠癌与直肠癌,治疗方案不固定等情况,BRAF和KRAS基因突变对术后结肠癌患者的预后影响尚存争议。因此,本项研究前瞻性从接受FOLFOX+/-西妥昔单抗辅助治疗的III结肠癌患者中收集生物标志。
研究方法:
研究人员对肿瘤BRAF V600E和KRAS外显子2基因突变进行分析,仅将MSS患者纳入组。将这些入组患者分为BRAF突变,KRAS突变,和双重野生型(WT)三组。采用一致性结果评估指标,在全组和各亚组中评估预后影响,然后将所有数据汇总。采用分层Cox比例风险模型对基因突变与复发间期(TTR),复发后生存期(SAR),和总生存期(OS)相关性进行分析。多变量模型对治疗和协变量(年龄,性别,肿瘤分级,T/N分期,肿瘤部位,ECOG PS)进行了校正。
研究结果:
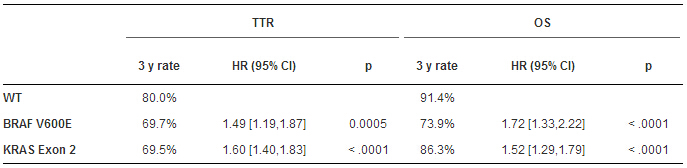
一共入组5577例,仅对3934例MSS患者评估BRAF和KRAS基因;其中279例BRAF突变,1450例KRAS突变,以及2205例双重WT。与WT组不同,突变两组的TTR和OS较短,且多因素分析也证实该结果(表)。WT组,KRAS突变组,BRAF突变组的中位SAR分别为2.57,2.09和1.0年,其中KRAS(HR:1.20-95%CI:1.01-1.44,P<0.0001),BRAF突变组(HR:3.01-95%CI:2.32-3.93,P<0.0001)。本项研究中治疗(联合或不联合西妥昔单抗)和KRAS/BRAF双突变的TTR(P值=0.38)和OS(P值=0.16)无明显差异。
结论:
本项研究通过一项大型III期术后结肠癌接受FOLFOX辅助治疗试验的汇总分析,发现BRAF V600E或KRAS 外显子2,包括密码子12或13突变,是TTR,SAR和OS的独立预测因子。在以后的临床试验辅助试验中应将这些突变纳入为重要分层因素。
会议专题》》》2015年ASCO年会专题报道
Prognostic value of BRAF V600E and KRAS exon 2 mutations in microsatellite stable (MSS), stage III colon cancers (CC) from patients (pts) treated with adjuvant FOLFOX+/- cetuximab: A pooled analysis of 3934 pts from the PETACC8 and N0147 trials.(Abstract 3507)
Authors:Julien Taieb, Karine Le Malicot,et al.
Session Type:Oral Abstract Session
Background: The prognostic value of BRAF and KRAS mutations in resected CC pts remains controversial due to published studies that include stage II & III, microsatellite instability (MSI) and MSS, colon and rectal tumors, and variable treatment regimens. We examined this question in prospectively collected biospecimens from MSS stage III CC pts receiving adjuvant FOLFOX +/- cetuximab.
Methods:Tumors were analyzed for BRAF V600E and KRAS exon 2 mutations, only MSS tumors were included. Three groups were defined: BRAF Mutant, KRAS Mutant and double wild-type (WT). The analytic strategy estimated study- and arm-specific prognostic effects to assess homogeneity of results, and then analysis of pooled data. Associations of mutations with time-to-recurrence (TTR), survival after relapse (SAR) and overall survival (OS) were analysed using a stratified Cox proportional hazards model. Multivariate models were adjusted for treatment and covariates (age, sex, tumor grade, T/N stage, tumor location, ECOG PS).
Results:Of the 5,577 pts enrolled, 3,934 tumors were MSS and evaluable for BRAF and KRAS; 279 pts were BRAF Mutant, 1,450 KRAS Mutant, and 2,205 WT. Both mutations were linked to shorter TTR and OS vs WT, and results were confirmed in multivariate analyses (table). Median SAR was 2.57, 2.09 and 1.0 year in WT, KRAS Mutant (HR: 1.20- 95%CI: 1.01-1.44, p < 0.0001) and BRAF mutant (HR: 3.01-95%CI: 2.32-3.93, p < 0.0001), respectively. No interaction was found between treatment (with or without cetuximab) and KRAS/BRAFmutations for TTR (p = 0.38) or OS (p = 0.16).
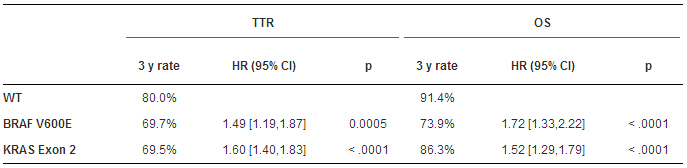
Conclusions:In a large pooled analysis of pts with resected stage III MSS colon cancers receiving adjuvant FOLFOX, BRAFV600E or KRAS exon 2 mutations, including codons 12 or 13, are independent predictors of significantly shorter TTR, SAR and OS. Future clinical trials in the adjuvant setting should consider these mutations as important stratification factors.




